Breaking the Silence with the PROBE Study.
Smarter Tracking
for Patients, Families, and Health Care Providers
By elevating the voice of patients, we can better assess the state of care around the world to help patients. PROBE was developed by patients, for patients, and collects information on the outcomes that are important to patients when they assess their health.
People with a bleeding disorder such as hemophilia should join our study. You can track your experience and receive personalized feedback. We want to hear from you.
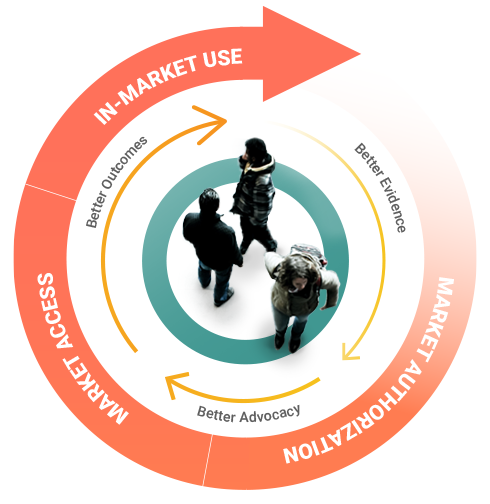
Better Advocacy
for NMOs, HTCs, and Bleeding Disorder Registries
PROBE empowers NMOs and patient organizations to advocate for and improve their healthcare environments. Secure linkages with registries provide a streamlined data collection platform.
Use PROBE to learn more about access to and quality of care in your country, and harness this information to jumpstart new programs and campaigns. Get in touch to see whether PROBE is available in your country and the many ways it can complement your work to achieve your organization's goals.
Unrivaled Access
for Drug Manufacturers, Research, and Academic Studies
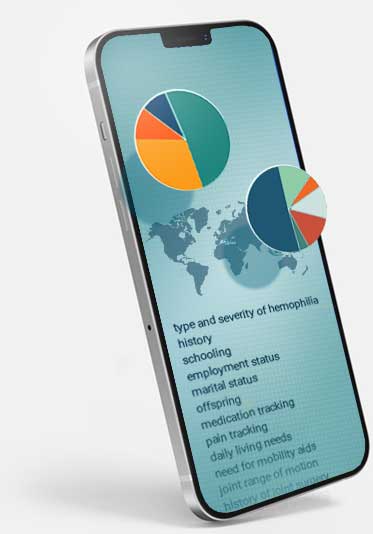
The PROBE study is a proven evidence-based methodology that reveals key data and insights about today’s patient experience in 100+ countries. PROBE is a validated instrument available in three formats to fit your needs.
PROBE is an established tool for collecting quality-of-life data in clinical studies, and its inclusion in a program provides a better understanding of the patient experience, which can help improve drug development, lower costs, mitigate risk and drive growth.
PROBE is an established research tool that prioritizes and amplifies the patient voice so we can better understand and improve the treatment and care of people around the world.
Who we serve
Why does the patient voice matter?
Patients are experts on living with their condition. Including the patient voice in research can help us define, collect, and report the patient journey more accurately and comprehensively. This patient-centered research allows for “Better Evidence.” It provides the kind of knowledge that:
• empowers patients and patient organizations
• improves outcomes collection
• speeds up the innovation life cycle
• ensures access to new treatments
• improves quality of life for patients

Thanks In Part To Our Study Funders
PROBE is funded with grant / research support from: